Hg l O2 g HgO s and we start to compare. Solid Al 2 SO 4 3.
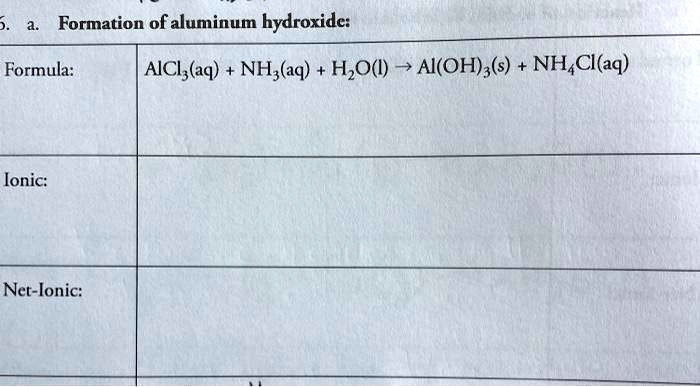
Solved Formation Of Aluminum Hydroxide Formula Aici Aq Nh Aq Hzo Ai Oh S Nh Cl Aq Ionic Net Ionic
First O atom number on both sides of the equation.

. We find 2 atoms on the right side and 1 in the left side so we put 2HgO instead of HgO to make the number of O equals on each side. Write a balanced chemical equation for the standard formation reaction of solid aluminum hydroxide Al OH. S Aln t Bt Ct 2 2 Dt 3 3 E 2t 2 G.
The ionic state of elements at 1 atm pressure and 298 K temperature are considered as an STP by the International Union of Pure and Applied Chemistry IUPAC. Thus I assume you mean the process that produces AlCl3s from the elemental states of the atoms. View the full answer.
C p heat capacity JmolK H standard enthalpy kJmol S standard entropy JmolK t temperature K 1000. The process also inhibits pepsin action by. 100 21 ratings Aluminium Hydroxide can be formed from Aluminium and Oxygen and Hydr.
So the equation become Hg. For example the most favorable interaction between a single monomer and if OH 4 was between AL OH 2 and SI OH 4 to form an ALSI2 with al. The published standard free-energy values for the common solid forms of aluminum hydroxide also disagree.
2Al OH 3 Al 2 O 3 3H 2 O. D- х. Work And Heat 63 Heat Of Reaction.
61 Energy And Its Units 62 First Law Of Thermodynamics. Write a balanced chemical equation for the standard formation reaction of solid aluminum hydroxide. The standard reaction of formation stands for the reaction in which the elements are present in the standard states.
Therefore the unbalanced standard formation reaction is. Two of these Which of the compounds below is an example of a network solid. Enthalpy Of Reaction 64 Thermochemical Equaitons 65 Applying Stoichiometry To Heats Of Reaction 66 Measuring Heats Of Reaction 67 Hesss Law 68 Standard Enthalpies Of Formation 69 Fuels-food Commercial Fuels And.
Experts are tested by Chegg as specialists in their subject area. Check the balance The thermal decomposition of aluminium hydroxide to produce aluminum oxide and water. The reaction for the formation of liquid titanium IV chloride will be.
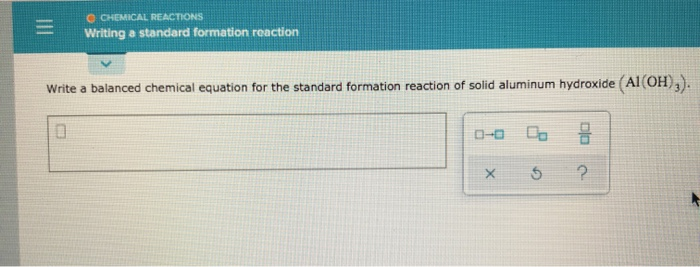
Since the pressure of the standard formation reaction is fixed at 1 bar the standard formation enthalpy or reaction heat is a function of temperature. Chemistry questions and answers. O THERMOCHEMISTRY Writing a standard formation reaction Write a balanced chemical equation for the standard formation reaction of solid aluminum hydroxide AI OH.
Al2 SO43 HCO3- 2Al OH3 SO4-- I do not want Al2 SO43 H2O 2Al OH3 H2SO4 I want to ensure that the reaction prefers or goes in. Example of solving the ALEKS problem Writing a standard formation reaction. The reaction for the.
Direct link to this balanced equation. H H 29815 At Bt 2 2 Ct 3 3 Dt 4 4 Et F H. We review their content and use your feedback to keep the quality high.
We review their content and use your feedback to keep the quality high. Chlorine naturally exists as a diatomic gas Cl2g at 25C and 1 atm. CHEMICAL REACTIONS Writing a standard formation reaction Write a balanced chemical equation for the standard formation reaction of solid aluminum hydroxide Al OH.
Standard state of sulfur is S 8 s Example Write the balanced chemical equation for the standard formation of. AluminumAcetate 3 3 AmmoniumHydroxide AluminumHydroxide 3 3 AmmoniumAcetate Reaction type. The change in enthalpy when 1 mole of the compound forms from its constituent elements in their standard states for methane CH 4 This value relates to the.
The chemical behavior of aluminum in dilute solutions presents some serious experimental difficulties. 100 4 ratings Transcribed image text. Experts are tested by Chegg as specialists in their subject area.
Who are the experts. A solid vanadium V oxide V 2 O 5 b solid aluminum hydroxide AlOH 3 Heat of formation Heat of formation. Double replacement Please tell about this free chemistry software to your friends.
This reaction takes place at a temperature of over 575C. The balanced chemical equation for the reaction between magnesium hydroxide and hydrochloric acid is MgOH2 2HCl --. Standard formation reaction of solid aluminum hydroxide.
The objective is to precipitate aluminum hydroxide per the following reaction which I believe to be correct. The formation of Aluminum Hydroxide includes the process of slowly dissolving in the stomach and then reacting with the hydrochloric acid which leads to the formation of aluminum chloride and water. Cl2g Als AlCl3s Balancing it to ensure that we form one mol of.
Check Save For Lates 2020 M A MacBook BO 44 14 3 7 2 4 6 8 0. Most reactions involving aluminum. Write a balanced chemical equation for the standard formation reaction of solid aluminum hydroxide AlOH3 Steel is considered to be an A.
Balanced Equation for aluminum and hydrochloric acid. Aluminum naturally exists at 25C and 1 atm as a solid.
Solved Write A Balanced Chemical Equation For The Standard Chegg Com

Aleks Writing A Standard Formation Reaction Youtube
Solved Write A Balanced Chemical Equation For The Standard Chegg Com
0 Comments